About the Phase 3 Clinical Trial for Tourette Syndrome
Emalex is conducting a Phase 3 clinical trial to evaluate the safety and efficacy of ecopipam (EBS-101) in children, adolescents and adults with Tourette’s Disorder (TD). This study is enrolling participants in North America and Europe and is regulated by the U.S. Food and Drug Administration (FDA) and by similar regulatory agencies outside the U.S.

About the Phase 3 Clinical Trial for Tourette Syndrome
Emalex is conducting a Phase 3 clinical trial to evaluate the safety and efficacy of ecopipam (EBS-101) in children, adolescents and adults with Tourette’s Disorder (TD). This study is enrolling participants in North America and Europe and is regulated by the U.S. Food and Drug Administration (FDA) and by similar regulatory agencies outside the U.S.

About the Phase 3 Clinical Trial for Tourette Syndrome
Emalex is conducting a Phase 3 clinical trial to evaluate the safety and efficacy of ecopipam (EBS-101) in children, adolescents and adults with Tourette’s Disorder (TD). This study is enrolling participants in North America and Europe and is regulated by the U.S. Food and Drug Administration (FDA) and by similar regulatory agencies outside the U.S.

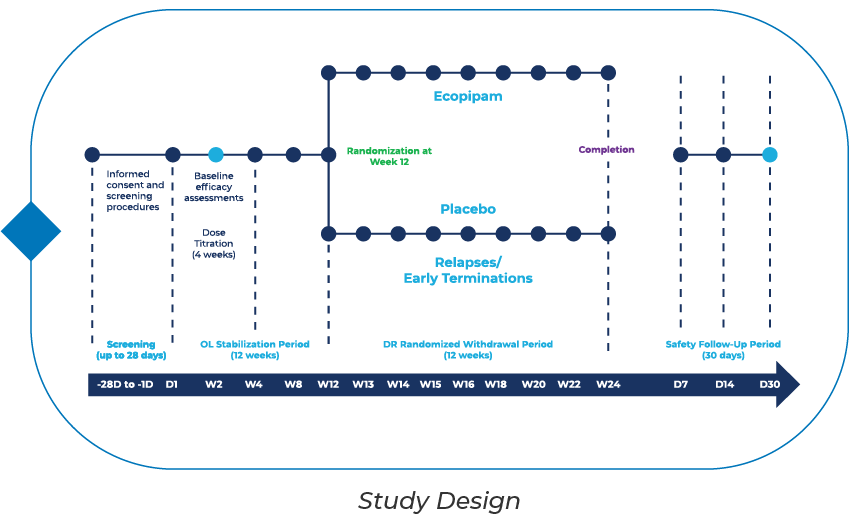
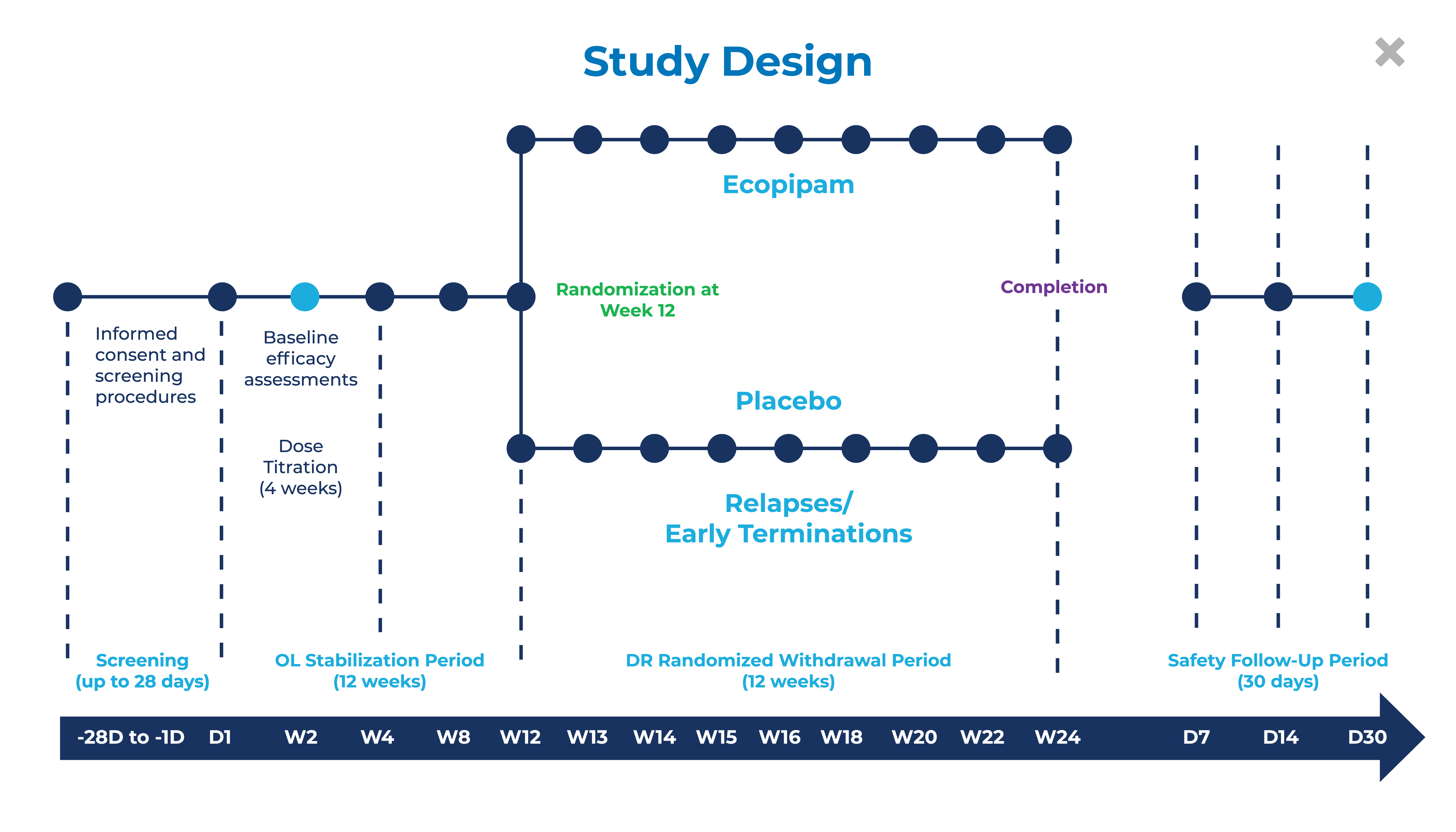
What Will the Study Involve?
- Screening Period – up to 28 days
- Open-Label Stabilization Period – comprised of a 4-week Titration Phase and 8-week Maintenance Phase.
- Double-Blind Randomized Withdrawal Period – subjects who respond to study treatment will be randomized 1:1 to either placebo or ecopipam for up to 12 weeks; subjects that meet relapse criteria will be discontinued.
- Safety Follow-Up Period – all subjects who enroll in the study will complete clinic visits at Days 7 and 14 after the last dose of study medication and a 30-day telephone follow up call.
- Additional study details should be discussed with healthcare providers at designated study sites.

What Will the Study Involve?
- Screening Period – up to 28 days
- Open-Label Stabilization Period – comprised of a 4-week Titration Phase and 8-week Maintenance Phase.
- Double-Blind Randomized Withdrawal Period – subjects who respond to study treatment will be randomized 1:1 to either placebo or ecopipam for up to 12 weeks; subjects that meet relapse criteria will be discontinued.
- Safety Follow-Up Period – all subjects who enroll in the study will complete clinic visits at Days 7 and 14 after the last dose of study medication and a 30-day telephone follow up call.
- Additional study details should be discussed with healthcare providers at designated study sites.

What Will the Study Involve?
- Screening Period – up to 28 days
- Open-Label Stabilization Period – comprised of a 4-week Titration Phase and 8-week Maintenance Phase.
- Double-Blind Randomized Withdrawal Period – subjects who respond to study treatment will be randomized 1:1 to either placebo or ecopipam for up to 12 weeks; subjects that meet relapse criteria will be discontinued.
- Safety Follow-Up Period – all subjects who enroll in the study will complete clinic visits at Days 7 and 14 after the last dose of study medication and a 30-day telephone follow up call.
- Additional study details should be discussed with healthcare providers at designated study sites.
Frequently Asked Questions
Frequently Asked Questions
Frequently Asked Questions